| Program | Indication | Preclinical | Phase 1 | Phase 2 | Phase 2b/3 |
|---|---|---|---|---|---|
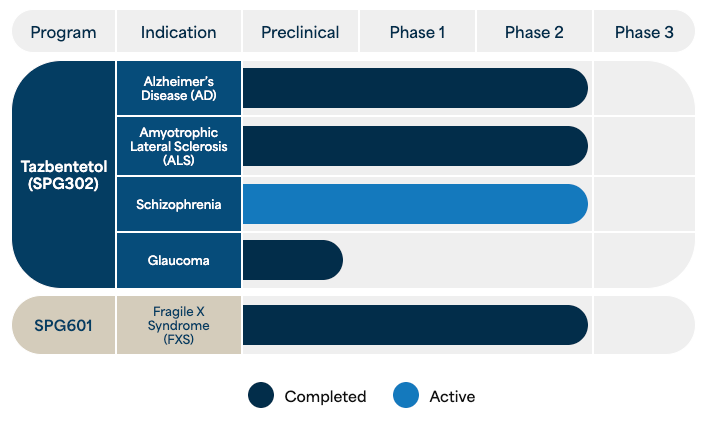
| Tazbentetol (SPG302) |
Alzheimer’s Disease (AD) |
||||
| Amyotrophic Lateral Sclerosis (ALS) |
|||||
| Schizophrenia (SCZ) |
|||||
| Glaucoma | |||||
| Undisclosed | |||||
| SPG601 | Fragile X Syndrome (FXS) |
||||
Completed
Active
Tazbentetol (SPG302)
- Alzheimer’s Disease (AD) (NCT06427668, NCT06833281) – the most common form of dementia worldwide
- Phase 2a completed with topline data presented at AAIC (July 2025), and CTAD conference (Dec. 2025)
- Rapid cognitive benefit vs placebo was shown within 4 weeks maintained through 40 weeks open label treatment
- Normalization of EEG signatures of aberrant brain activity in AD supporting pharmacodynamic activity
- Amyotrophic Lateral Sclerosis (ALS) (NCT05882695, NCT06903286) – the most common adult-onset motor neuron disease
- Phase 2a completed with topline data presented at NEALS (Oct. 2025) and MNDA (Dec. 2025)
- Rapid and significant EEG correction in ALS brain power signature preceded and correlated with functional benefits (ALSFRS-R)
- FDA & EMA Orphan designation in ALS
- Schizophrenia (SCZ) (NCT06442462) – the most prevalent psychotic disorder worldwide
- Phase 2 trial has completed enrollment in AUS and the USA with projected top-line results in 1H2026
- Glaucoma (Pre-Clinical)
- Undisclosed (Pre-Clinical)
SPG601
- Fragile X Syndrome (FXS) (NCT06413537) – the most common cause of intellectual disability and autism
- Phase 2a (single dose cross-over design) completed, results presented at AACAP
- Significant improvement in signature EEG power abnormalities
- Significant improvement in selective attention task and evidence of change in other components of the NIH Toolbox
- Orphan Designation | Fast Track | EMA Orphan Designation