Reversing Synapse Loss Starts Today
For those with neurodegenerative diseases
this could be a game changer.
SPINOGENIX is pioneering first-in-class therapeutics that restore synapses to improve the lives of patients worldwide.
Unique Approaches for Conditions Involving the Loss or Dysfunction of Synapses

Patent-protected compound
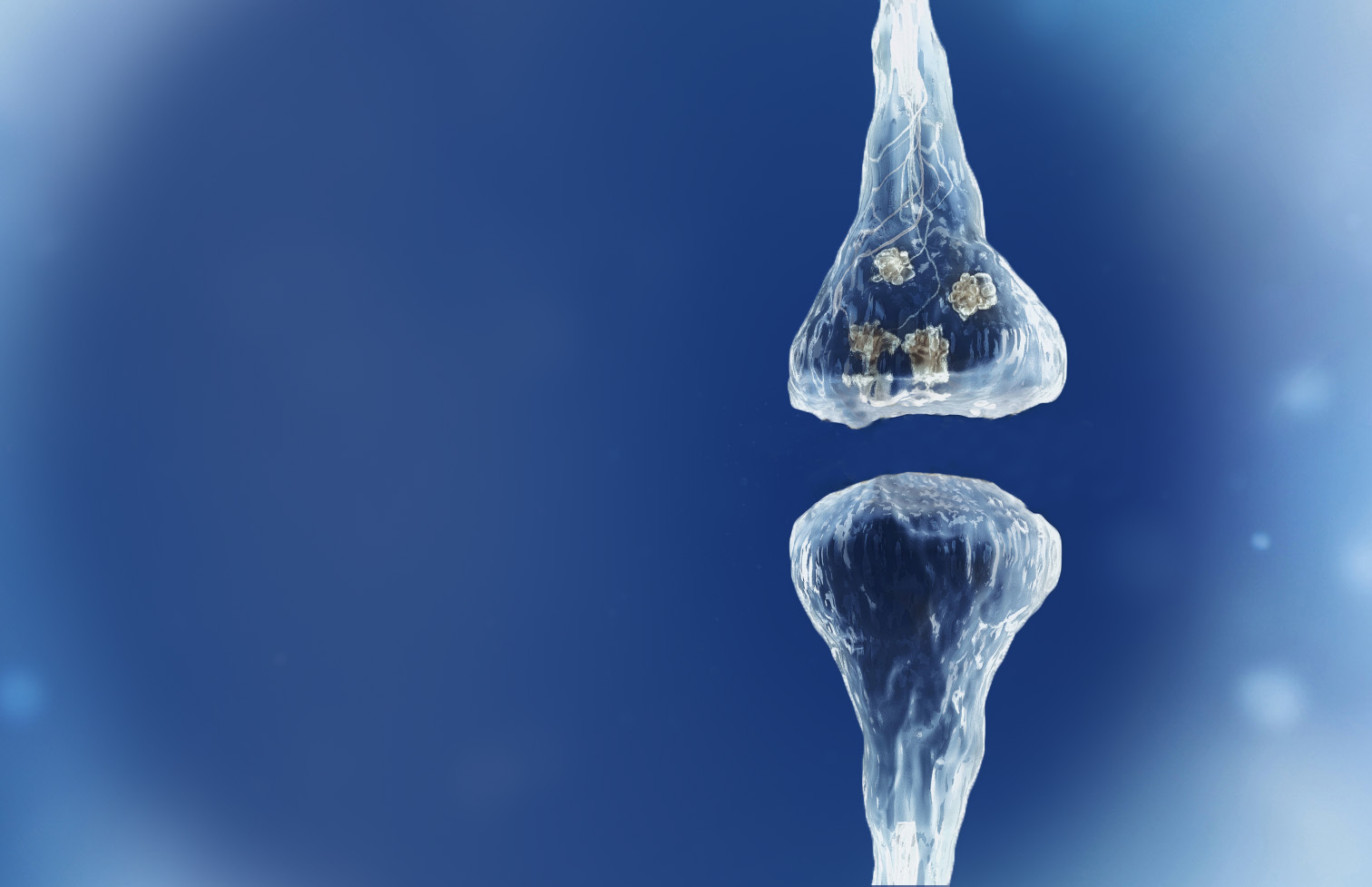
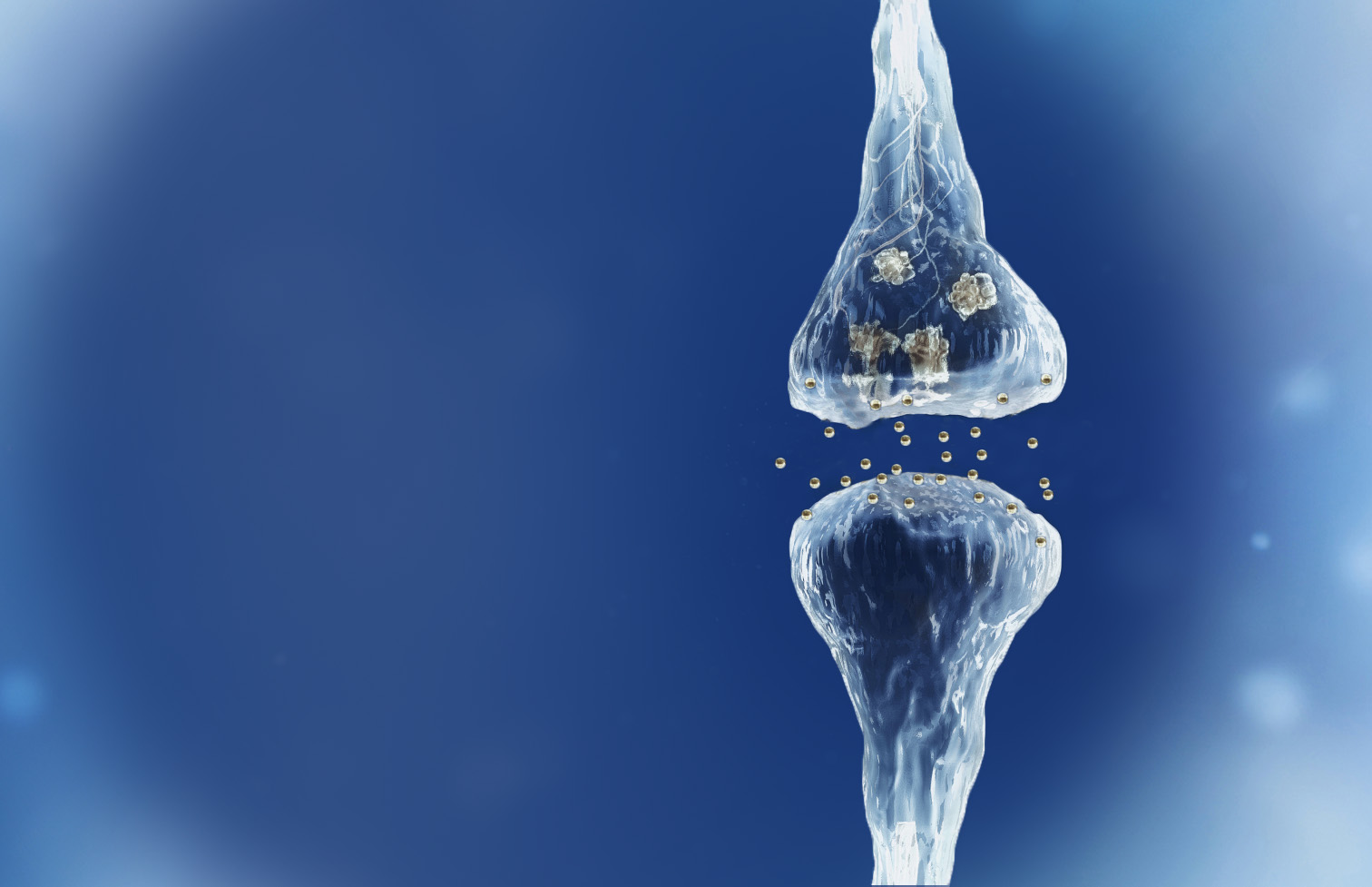
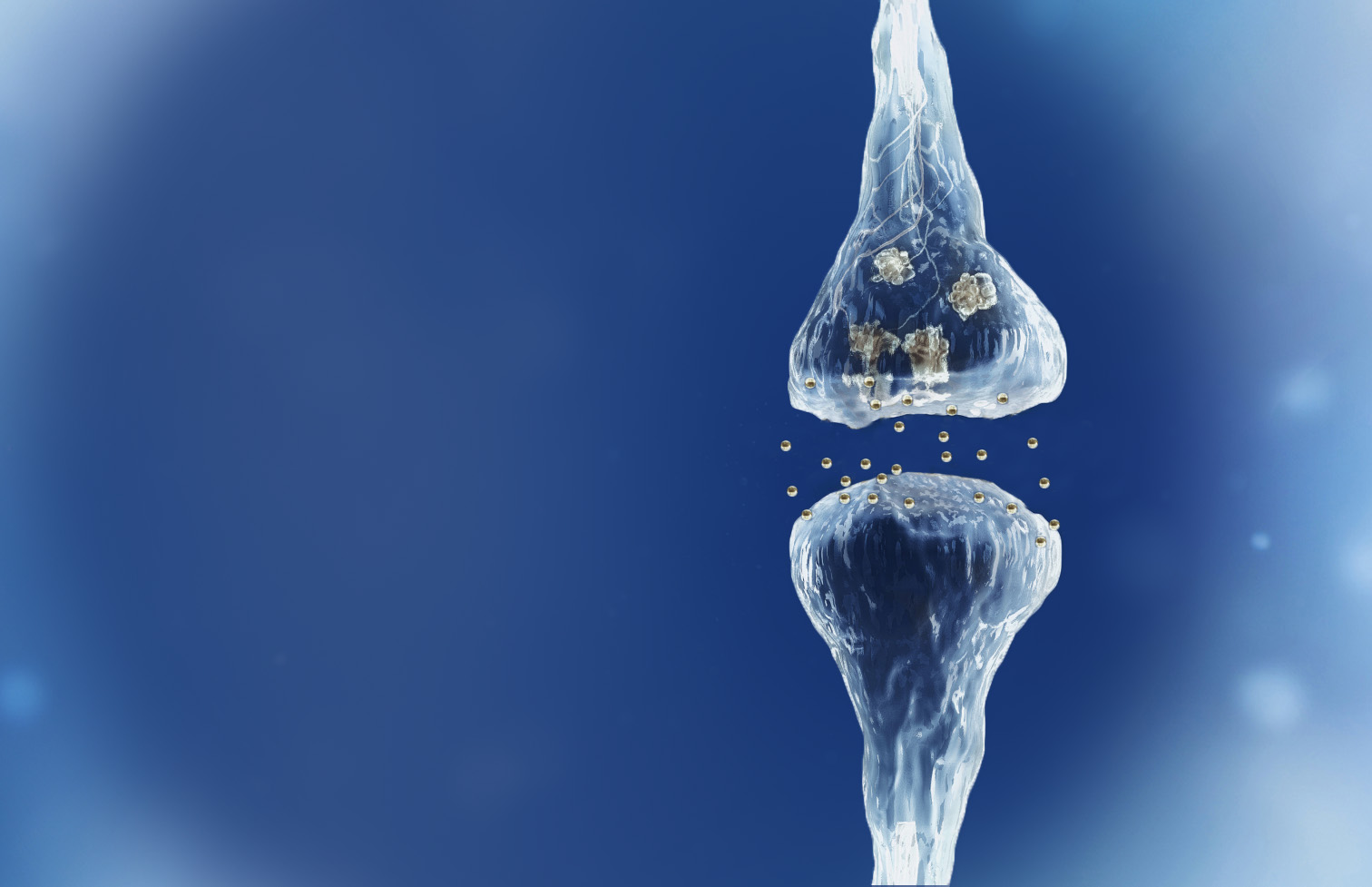
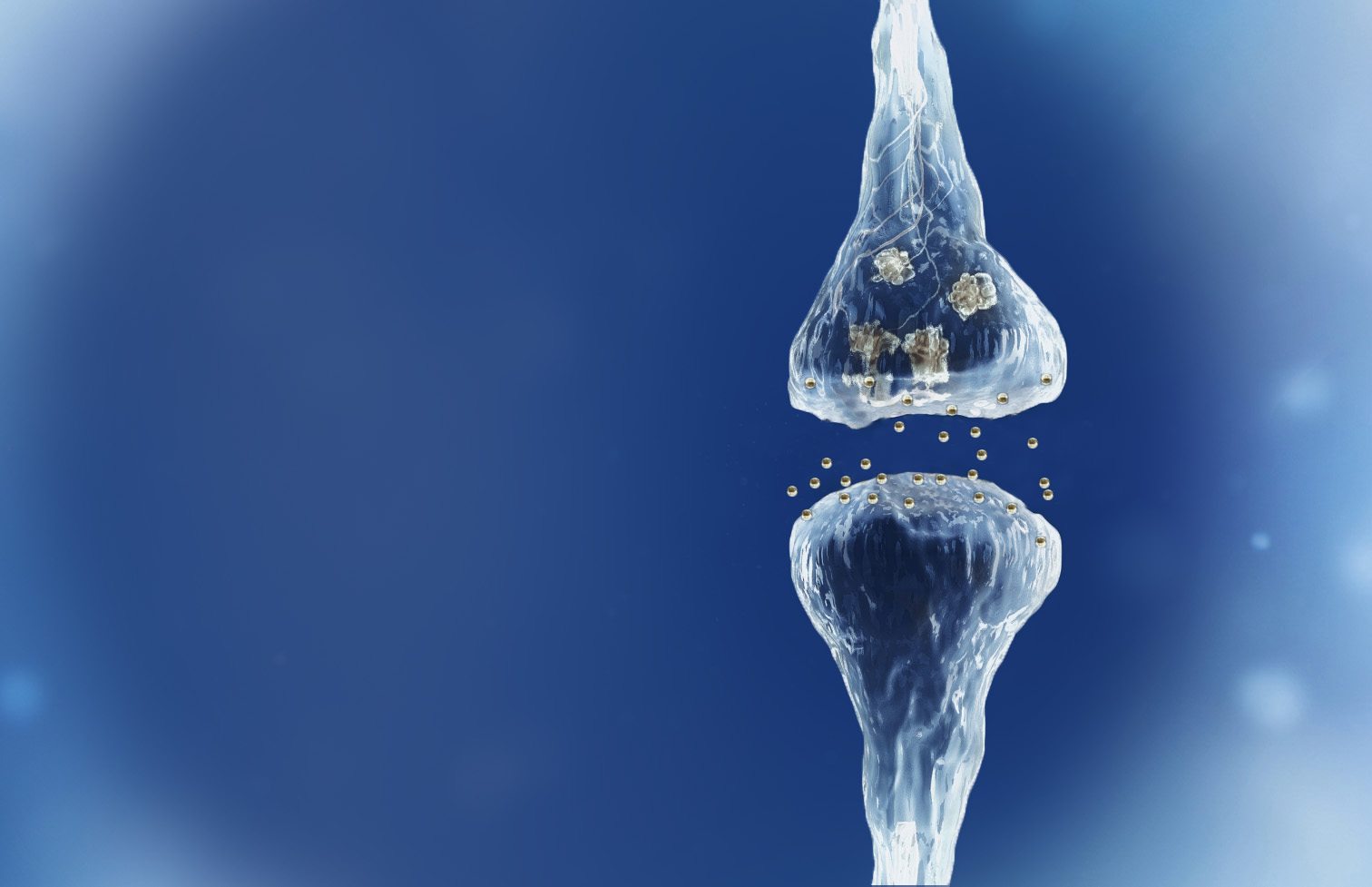
Spinogenix has designed small molecules to help restore the brain connections (synapses) in neurodegenerative, neuropsychiatric and neurodevelopmental conditions including amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, schizophrenia, Fragile X syndrome and many others.
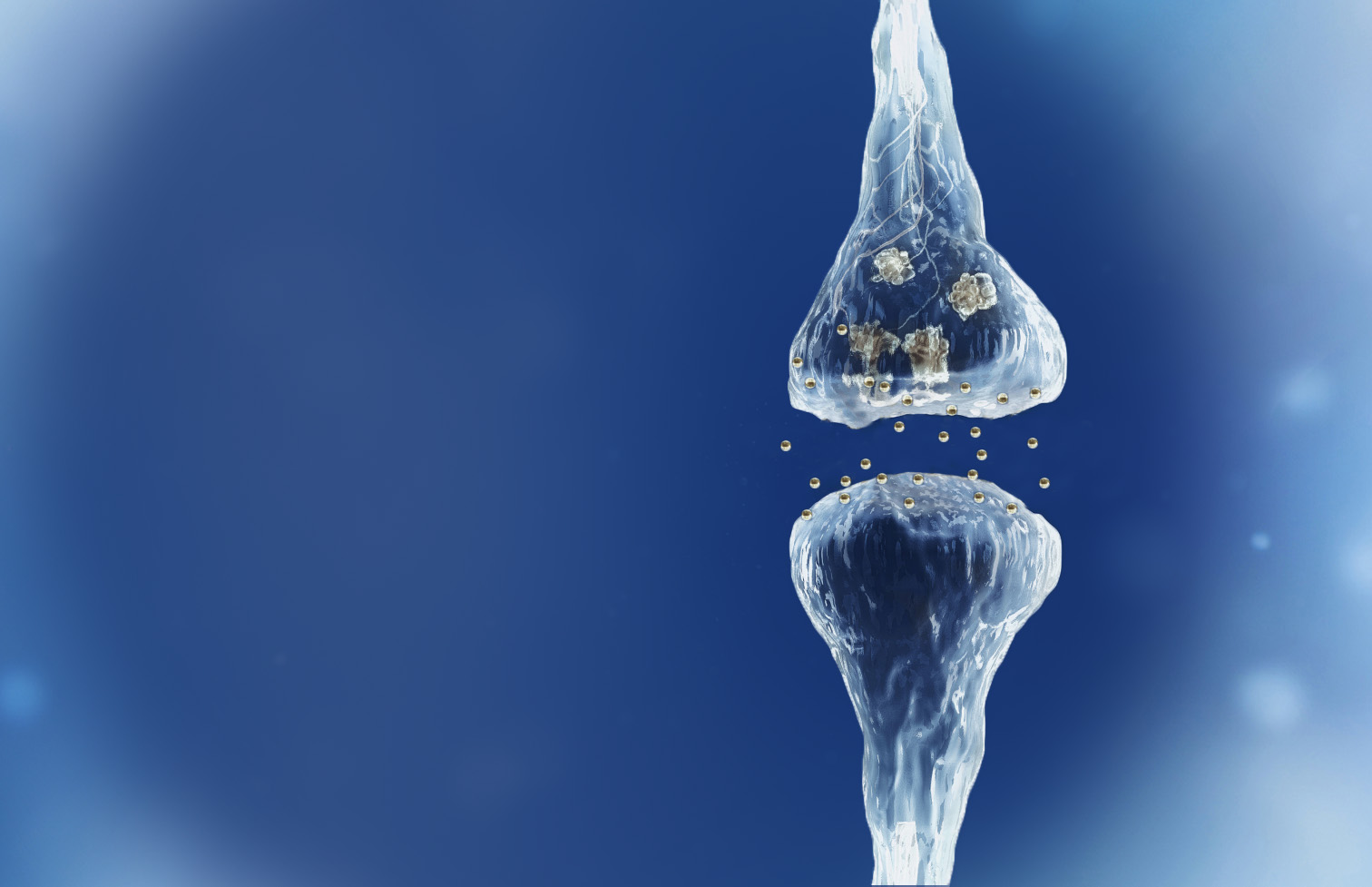
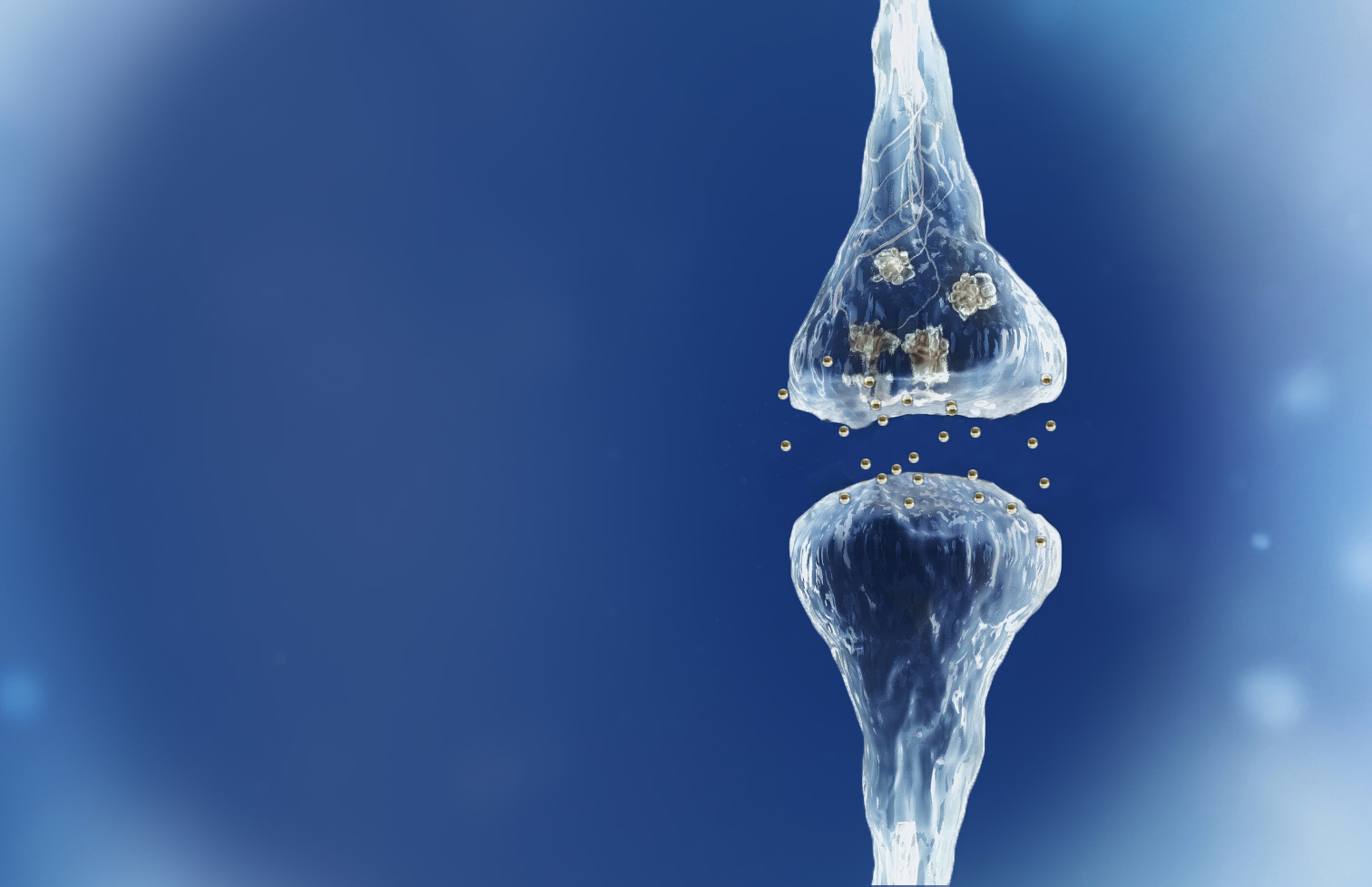
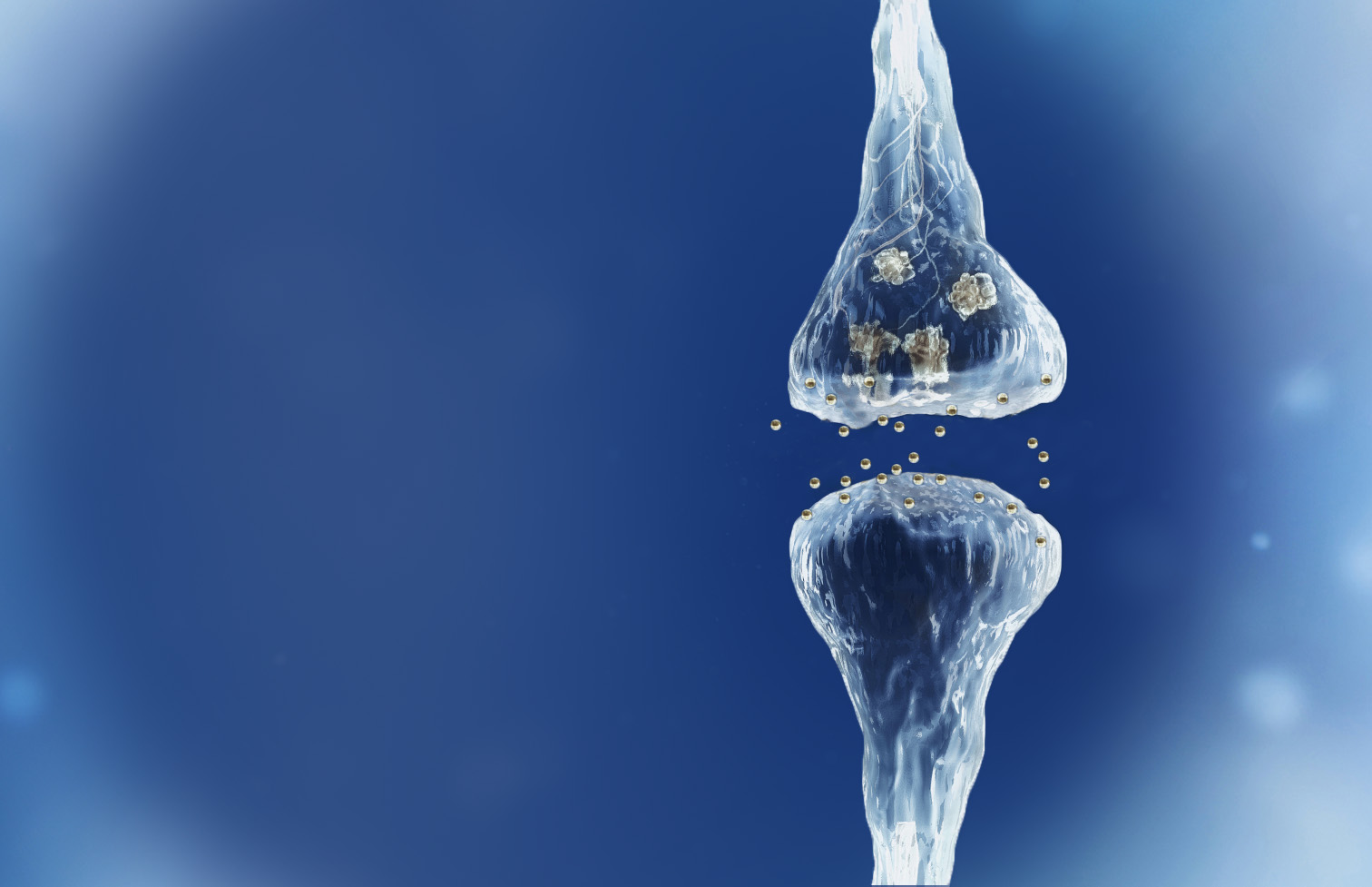
Reverse of the synaptic degeneration process
Spinogenix has demonstrated SPG302 as an innovative treatment for regenerating synapses to reverse declines in cognitive and motor function, which is fundamentally different from other therapeutics that aim to slow degeneration.
Once-a-day tablet
Spinogenix has developed SPG302 as a once-a-day tablet, which is significantly different from recently approved antibody therapies for Alzheimer’s disease that require IV infusion by a healthcare professional.
Regenerative Platform Approach
Multiple NIH and DOD grants have been awarded to support our unique treatment for Alzheimer’s and ALS patients. This approach leverages our TAGS™ technology to trigger the formation of new synaptic connections, in effect regenerating synapses that have been lost.
Current treatments leave a critical gap in effective and patient-friendly solutions
Our unique approach aims to improve patient outcomes while also avoiding burdens imposed by many current therapies for conditions involving synapse loss.

SPG302 Clinical Trials in ALS, Alzheimer’s Disease, and Schizophrenia
There are no approved therapies that regenerate synapses in Amyotrophic lateral sclerosis (ALS), Alzheimer’s Disease, and Schizophrenia. SPG302 is the first synaptic regenerative therapy to be tested that aims to restore cognitive, motor, and other functions in these diseases.
ALS
Spinogenix’s first-in-human Phase 1/2 study in Australia is fully enrolled and not recruiting.
Alzheimer’s Disease
The Australia Human Research Ethics Committees has approved the initiation of Spinogenix’s Phase 2 study in Australia to evaluate the safety, tolerability, clinical efficacy, pharmacokinetics, and pharmacodynamics of SPG302 in adult participants with mild-to-moderate Alzheimer’s disease.

SPG601 Clinical Trial in Fragile X Syndrome
The U.S. FDA has approved Spinogenix’s IND application for SPG601 for Fragile X Syndrome (FXS) and granted Orphan Drug Designation for SPG601 for the treatment of FXS. FXS is the leading inherited form of intellectual disability and known cause of autism for which no FDA-approved treatments exist.
Spinogenix’s Phase 2a clinical trial is evaluating the neurophysiological and clinical effects of single-dose SPG601 and placebo in adult men with FXS.
Additional information can be found on ClinicalTrials.gov (NCT06413537).
To my knowledge, this is the first clinical trial in ALS focused on regenerating synapses with a small molecule. It has the potential to be used in combination with many other treatments approved and in development.
-Dr. Merit cudkowicz, director of the Sean m. healey &
amg center for ALS at massachusetts general hospital



