Reversing Synapse Loss Starts Today
For those with neurodegenerative diseases
this could be a game changer.
SPINOGENIX is pioneering first-in-class therapeutics that restore synapses to improve the lives of patients worldwide.
Unique Approaches for Conditions Involving the Loss or Dysfunction of Synapses

Patent-protected compound
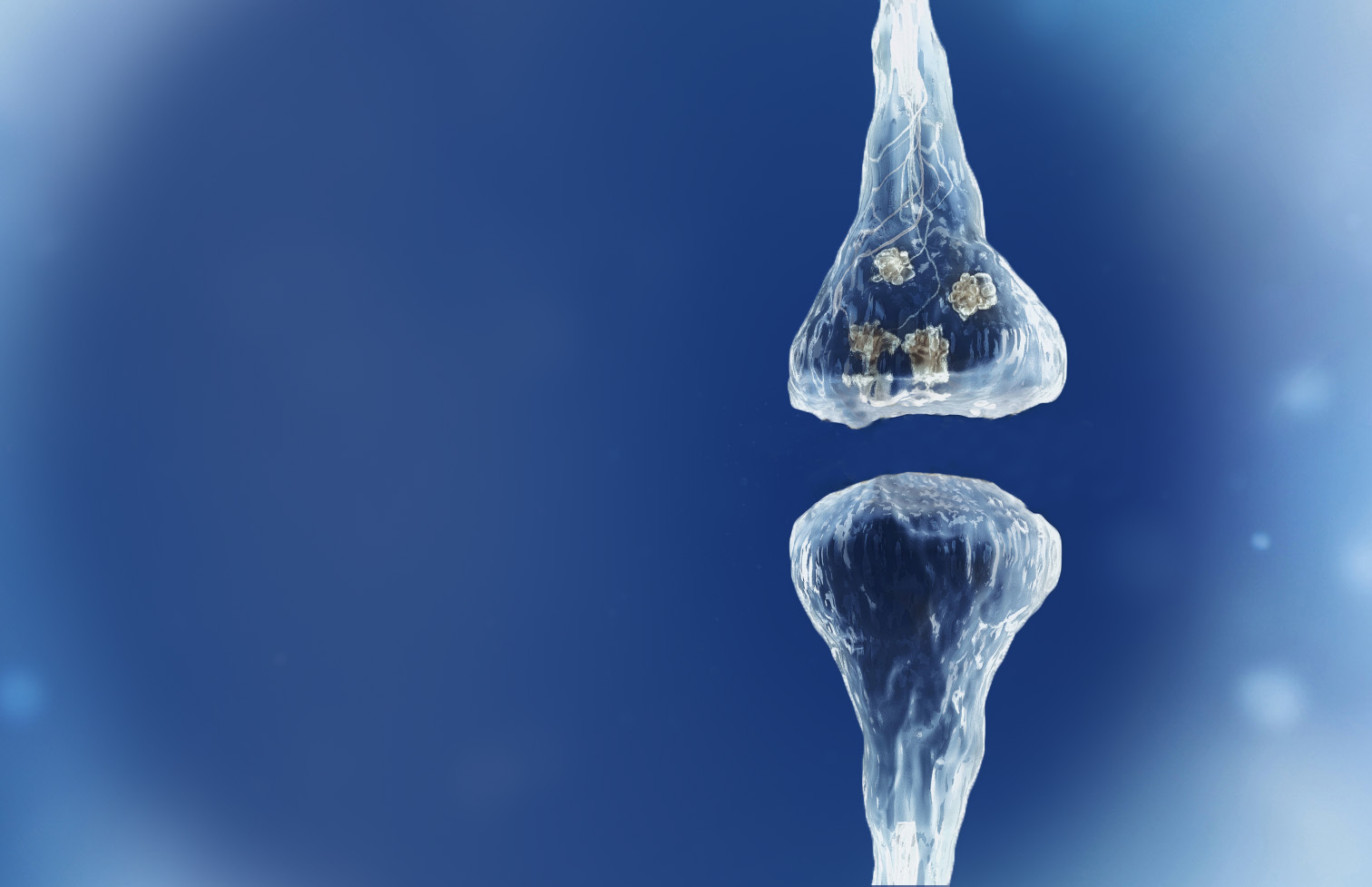
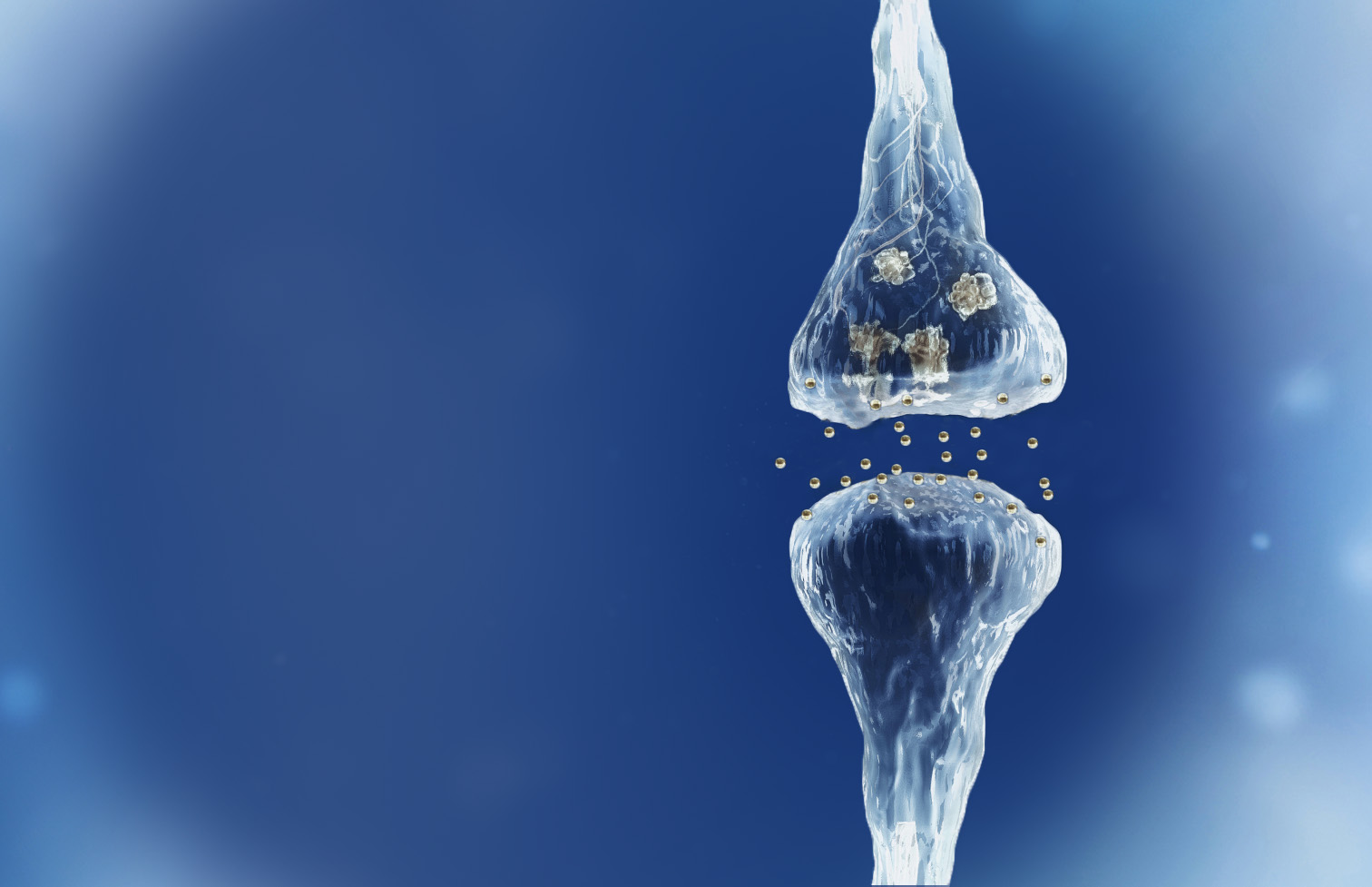
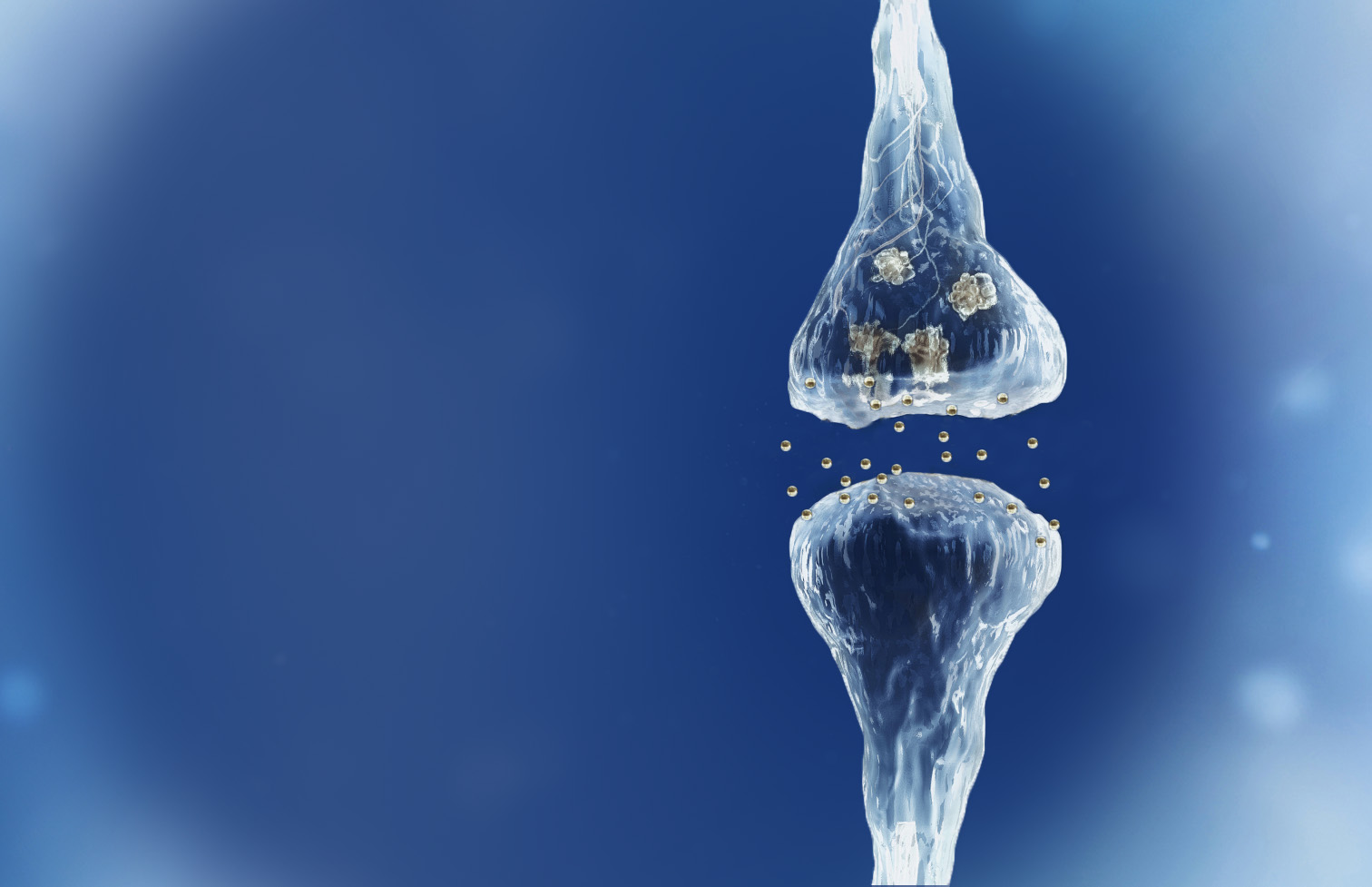
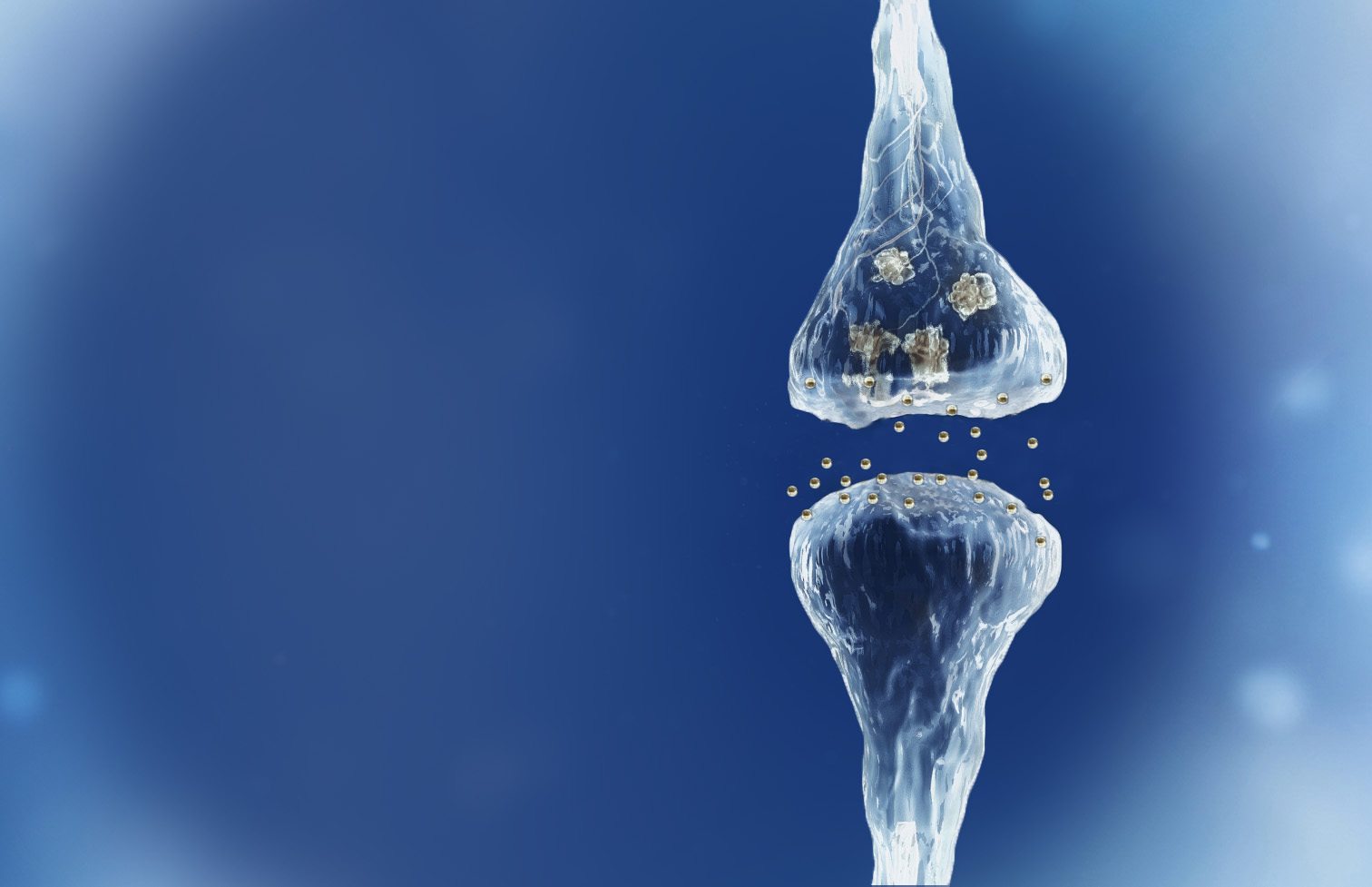
Spinogenix has designed small molecules to help restore the brain connections (synapses) in neurodegenerative, neuropsychiatric and neurodevelopmental conditions including amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, schizophrenia, Fragile X Syndrome and many others.
Reverse of the synaptic degeneration process
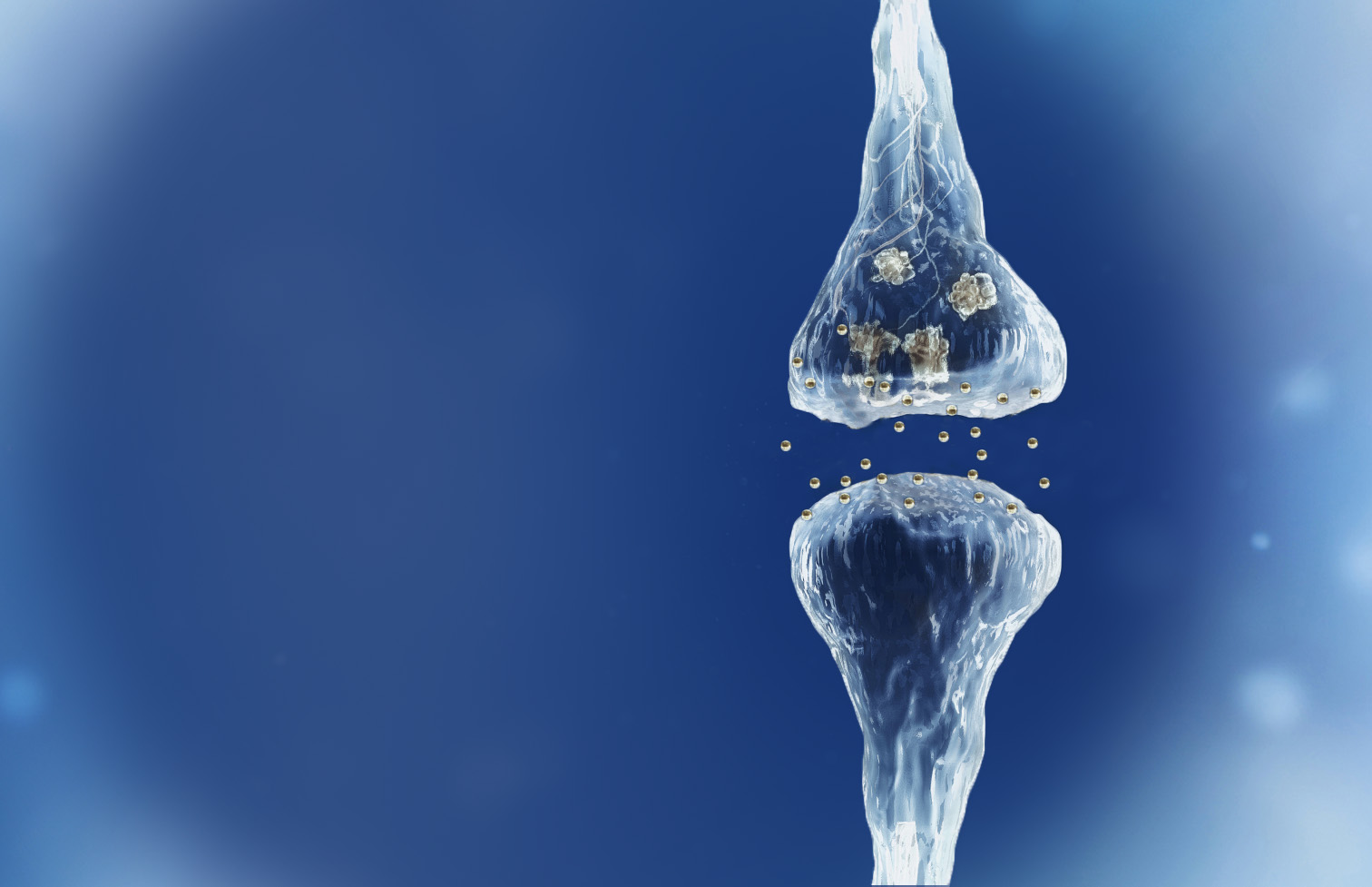
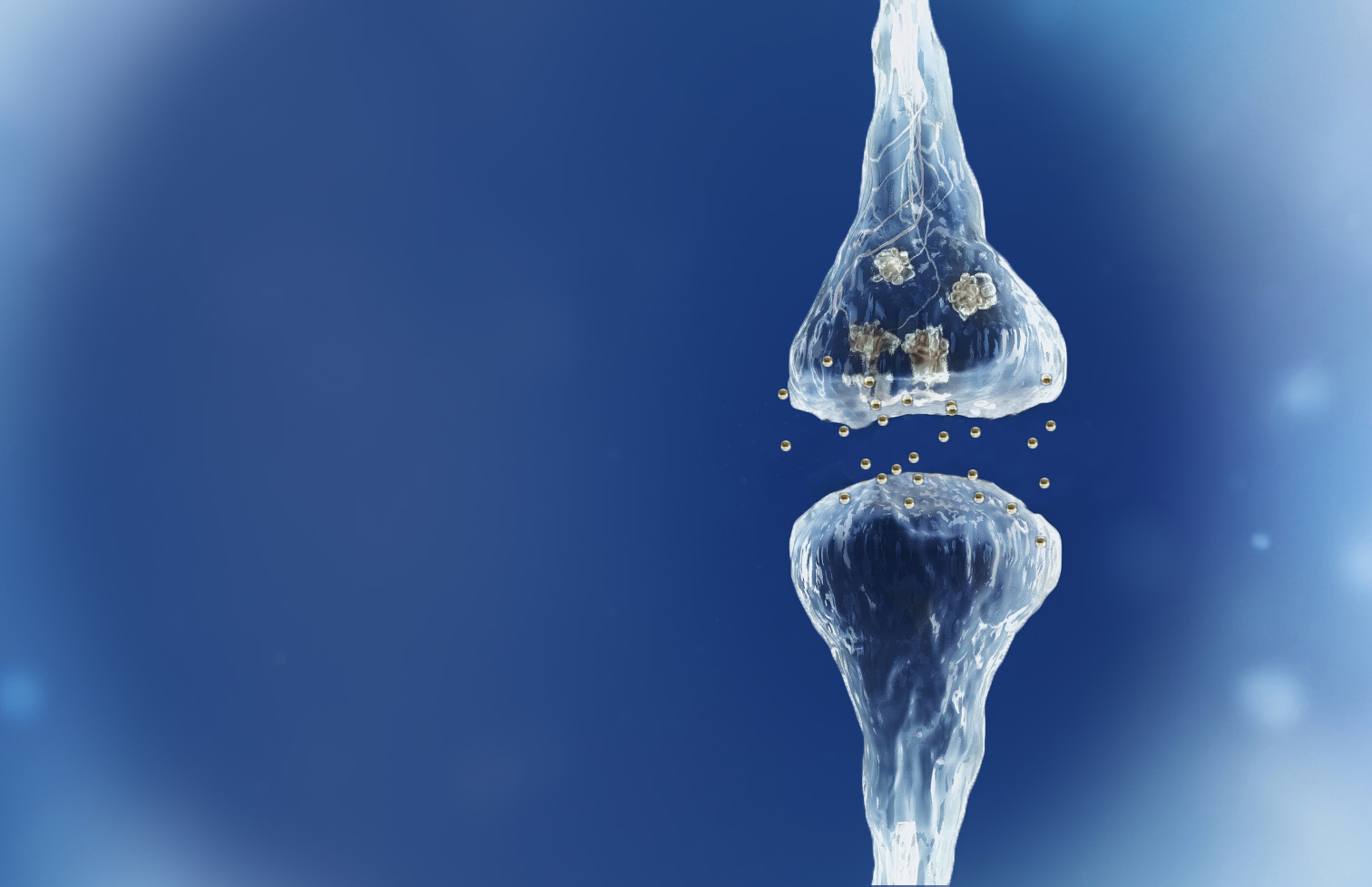
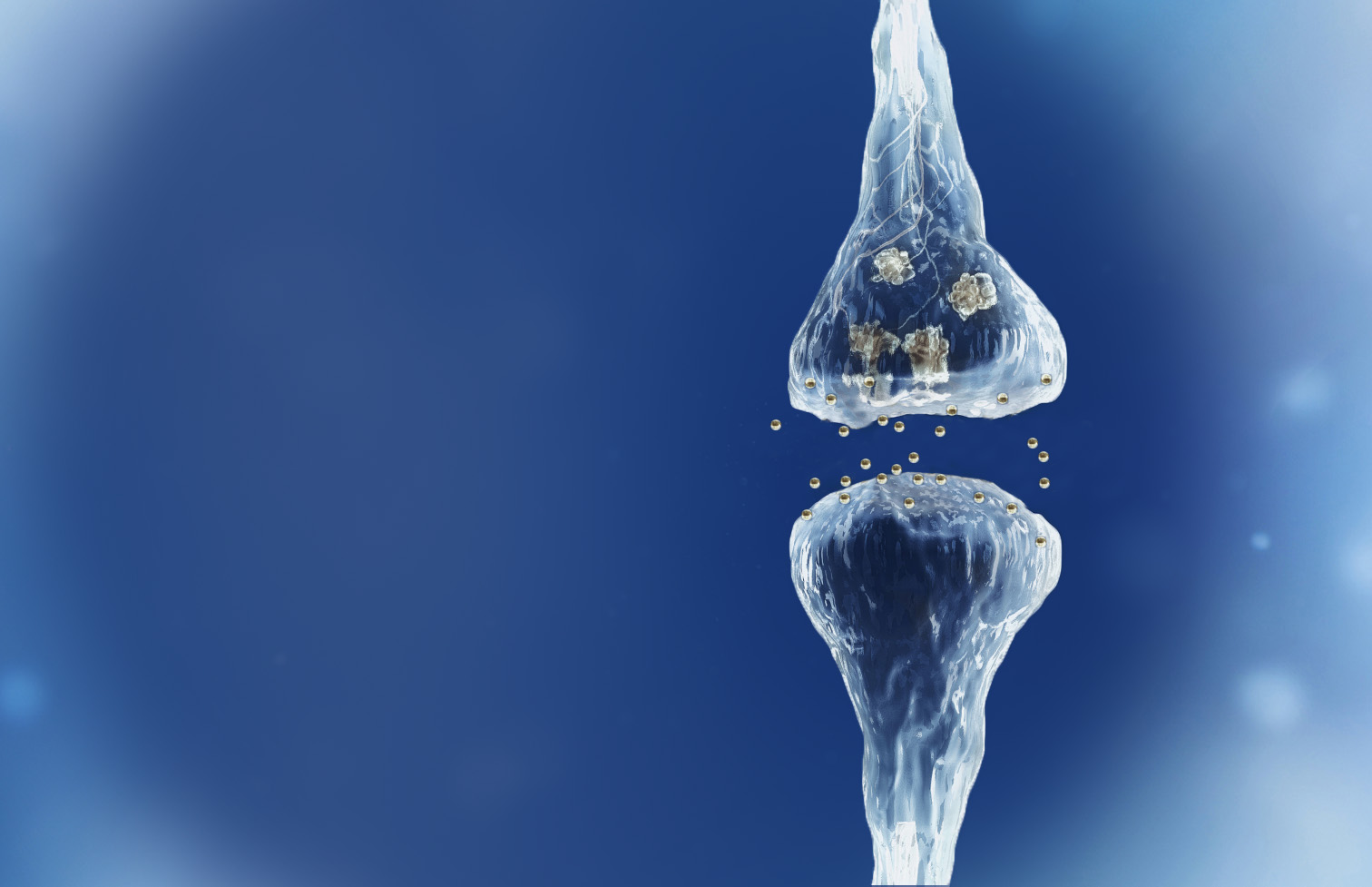
Spinogenix has demonstrated SPG302 as an innovative treatment for regenerating synapses to reverse declines in cognitive and motor function, which is fundamentally different from other therapeutics that aim to slow degeneration.
Once-a-day tablet
Spinogenix has developed SPG302 as a once-a-day tablet, which is significantly different from recently approved antibody therapies for Alzheimer’s disease that require IV infusion by a healthcare professional.
Regenerative Platform Approach
Multiple NIH and DOD grants have been awarded to support our unique treatment for Alzheimer’s and ALS patients. This approach leverages our TAGS™ technology to trigger the formation of new synaptic connections, in effect regenerating synapses that have been lost.
Current treatments leave a critical gap in effective and patient-friendly solutions
Our unique approach aims to improve patient outcomes while also avoiding burdens imposed by many current therapies for conditions involving synapse loss.

SPG302 Clinical Trials in ALS, Alzheimer’s Disease, and Schizophrenia
SPG302 is the first synaptic regenerative therapy to be tested in ALS, and Spinogenix is aiming to make it the first such therapy in Alzheimer’s and schizophrenia.
Spinogenix’s first-in-human Phase 1 study in Australia is currently underway to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of SPG302 in healthy volunteers and ALS patients. Additional information can be found on ClinicalTrials.gov(NCT05882695) Discussions for expansion of SPG302 into Phase 2 studies in Australia for Alzheimer’s Disease and schizophrenia are currently ongoing.
The U.S. Food and Drug Administration (FDA) has granted SPG302 Orphan Drug Designation in ALS. Spinogenix has successfully completed pre-IND (Investigational New Drug) interactions with the FDA regarding the current plan for SPG302 in the U.S.

SPG601 Clinical Trial in Fragile X Syndrome
The U.S. Food and Drug Administration (FDA) has approved Spinogenix’s Investigational New Drug (IND) application for SPG601 for Fragile X Syndrome (FXS). FXS is the leading inherited form of intellectual disability and known cause of autism for which no FDA-approved treatments exist.
Spinogenix’s Phase 2a clinical trial will evaluate the neurophysiological and clinical effects of single-dose SPG601 and placebo in adult men with FXS.
To my knowledge, this is the first clinical trial in ALS focused on regenerating synapses with a small molecule. It has the potential to be used in combination with many other treatments approved and in development.
-Dr. Merit cudkowicz, director of the Sean m. healey &
amg center for ALS at massachusetts general hospital



